Point of Care
Nursing Reference Center Plus is no longer available. The publisher is creating a new resource called Nursing & Allied Health Reference Center. Stay tuned for launch date.
Practice Guidelines
Asking an Answerable Question
Using PICO TO Structure a Clinical Question
The PICO model offers one way to construct your question:
| P = Patient or population | Identify the patient groups and medical problem central to your question |
| I = Intervention | Which intervention is being studied? |
| C = Comparative Intervention | Which comparison interventions are being considered (if any)? |
| O = Outcome | What would you like to measure or improve? e.g. reduced mortality, improved quality of life |
Let's look at an example:
Situation: One of your area's goals is to reduce occurrence and improve treatment of bed sores. Dynamic bed support surfaces are being discussed.
This can be written into PICO categories:
P = Patients with pressure ulcers
I = Dynamic bed support surfaces
C = Static bed support surfaces
O = fewer pressure ulcers
From this we can state the answerable research question as: "For patients with pressure ulcers do dynamic bed support surfaces, when compared with static bed support surfaces, result in fewer pressure ulcers?"
What Study Designs Answer Which Questions?
Appraising Therapy Studies
1. VALIDITY
Think FRISBE
|
F patient Follow-up |
|
|
R Randomization |
|
|
I Intention to treat |
|
|
S Similar baseline characteristics |
|
|
B Blinding |
|
|
E Equal treatment |
|
2. CLINICAL IMPORTANCE
ARR Absolute Risk Reduction
The difference between the risk of an event without the treatment and the risk of an event with the treatment.
EER-CER=ARR
CER Control Event Rate
The risk of an event happening in the control / non-treatment group.
EER Experimental Event Rate
The risk of an event happening in the experimental / treatment group.
NNT Number Needed to Treat
The number of patients that would need to be treated in order to prevent one event.
1/ARR=NNT
RR Relative Risk / Risk Ratio
The ratio of the difference between the risk of an event without the treatment and the risk of an event with the treatment: 50% greater risk, 5 times the risk, etc.
EER/CER=RR
RRR Relative Risk Reduction
The ratio of the number fewer events in the experimental / treatment group compared to the control group
(EER-CER)/CER=RRR
Appraising Diagnosis Studies
1. VALIDITY
2. CLINICAL IMPORTANCE
Sensitivity (Sn)
The percentage or proportion of people that have the condition AND test positive with the experimental test.
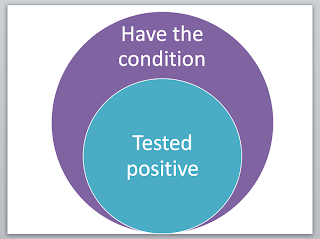
Specificity (Sp)
The percentage or proportion of people that DO NOT have the condition AND DO NOT test positive with the experimental test.

Positive Predictive Value (PPV)
The percentage or proportion of people that test positive with the experimental test who have the condition

Negative Predictor Value (NPV)
The percentage or proportion of people that do not test positive and do not have the condition.

Appraising Systematic Reviews
VALIDITY
